Abstract
BCL2 family proteins represent attractive and druggable targets for hematologic malignancy. Pro-survival proteins BCL2, BCL-XL, and MCL1 are key targets of BH3 mimetic and inhibitory drugs that are in clinical trials and/or clinical use for hematologic malignancies. Although the mechanism by which BH3-mimetic drugs bind anti-apoptotic family members is well-understood, less is known about off-target impacts of these drugs across the phenome. Furthermore, the impact of other BCL2 family proteins might have on clinically relevant phenotypes has not been explored. The development of and implementation of drugs that modulate the function of BCL2 family proteins could be enhanced by understanding gene-specific impacts on human phenotypes. Understanding the phenomic impacts of modulating BCL2 family expression has potential to enhance efficient development and inform clinical investigation of BCL2 modulating therapeutics for both hematologic and non-hematologic malignancy by identifying novel phenotype associations as well as by identifying potential side effects. In this study, we leveraged population-level differences in genetically determined gene expression of eighteen BCL-2 family genes to identify phenotypes that associate with altered genetically determined expression.
We performed transcriptome-wide association studies (TWAS) utilizing GWAS summary statistics for 357 unique ICD-10 CM diagnosis codes and 31 unique hematologic traits from the UK Biobank V3 release (UKBB v3; n ~500,000). Summary statistics-based TWAS was applied using tissue-specific weights generated by Joint Tissue Integration (JTI) modeling of all 49 GTEx v8 tissues (Zhou et al., Nature Genetics 2020). From there, significant associations with eighteen core BCL2-family genes were pulled for analysis. This approach identified 25 unique phenotypes associated with expression of BCL2 family genes, given a Bonferroni-adjusted significance cutoff of p=1.5e-07.
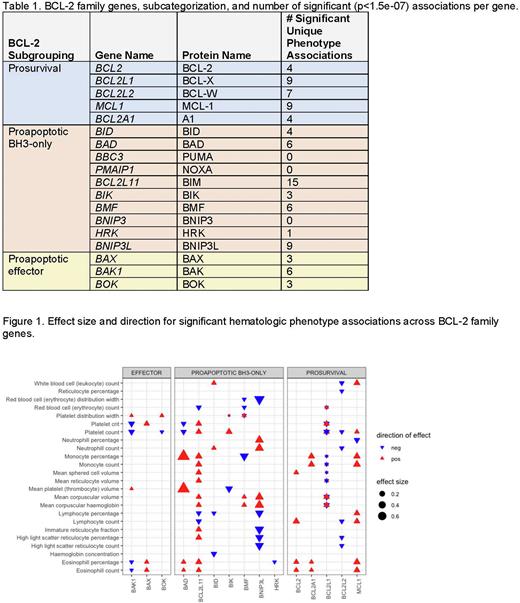
Gene-phenotype associations (Table 1) were remarkably enriched for hematologic phenotypes derived from complete blood count data, and demonstrated considerable overlap between cell death genes, with only three of these phenotypes uniquely mapping to a single gene. Conversely, BCL2 family genes were significantly enriched in hematologic trait datasets (p<0.01). BCL2L11, encoding BIM, maps to the most unique phenotypes (n=15), while BCL2 and BAX, which are highly conserved, have only four and three unique phenotype associations respectively. BBC3, PMAIP1, and BNIP3 did not have significant associations, suggesting that modulating genetically-determined expression levels of these genes alone contributes modestly at most to changes in individual human phenotypes. BCL2L1, which encodes BCL-X, exhibits associations with bi-directional effect sizes across different tissues (Figure 1). This is consistent with the known divergent biological functions of long versus short isoforms of this gene product (BCL-xL and BCL-xS). Though BCL-2 family members are thought to have redundant functions across members within the same subclassification, (e.g. BH3-only, pro-survival, effector), not all genes within these subclasses consistently exhibited the same phenotype associations. More strikingly, genes within the same class that shared phenotype associations did not always exhibit the same directionality of effect, suggesting that these genes may have divergent roles contributing to these phenotypes.
Overall, our findings support the premise that BCL2-family gene expression distinctively targets hematologic phenotypes. The results also suggest that genes with canonically "conserved” functions may have biologically unique contributions to hematopoiesis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.